We are developing bemnifosbuvir in combination with ruzasvir for the treatment of HCV. The combination of bemnifosbuvir and ruzasvir has the potential to improve upon the current standard of care by offering a short duration, pangenotypic, protease inhibitor-free treatment for patients with HCV, with or without cirrhosis. As single agents, both bemnifosbuvir and ruzasvir have demonstrated potent pan-genotypic antiviral activity against HCV.
Based on our preclinical and clinical data to-date, the combination of bemnifosbuvir and ruzasvir has the potential to offer the following potential benefits:
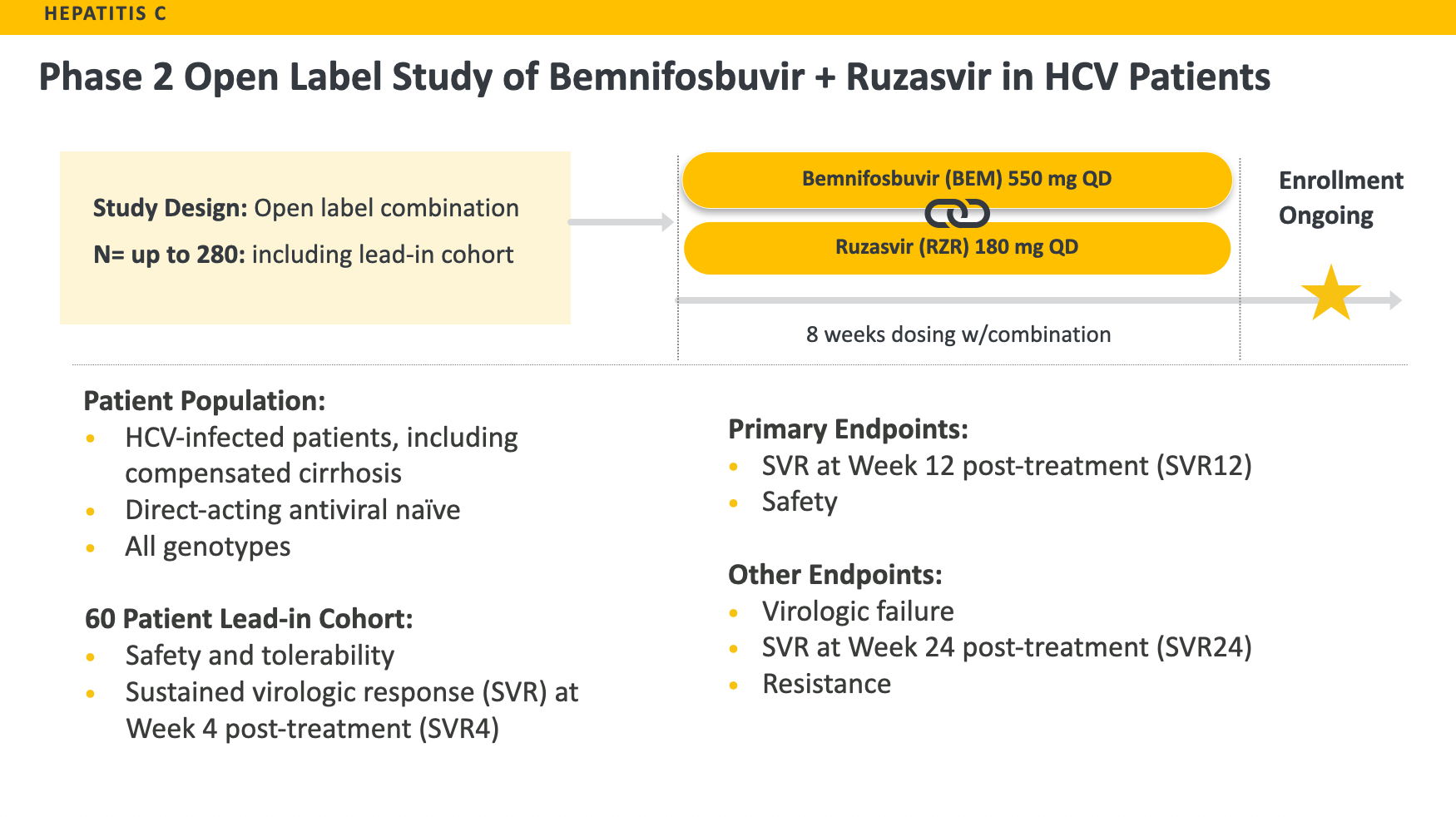
We are currently conducting a global Phase 2 clinical trial of bemnifosbuvir in combination with ruzasvir, an oral NS5A inhibitor, in treatment-naïve, HCV-infected patients either without cirrhosis or with compensated cirrhosis. This study is designed to evaluate the safety and efficacy of eight weeks of treatment with the combination consisting of once-daily bemnifosbuvir 550 mg and ruzasvir 180 mg. Up to approximately 280 HCV-infected, treatment-naïve patients across all genotypes (GT), including the lead-in cohort of 60 patients without cirrhosis, are expected to be enrolled in this Phase 2 clinical trial.
The primary endpoints of the study are safety and sustained virologic response (SVR) at Week 12 post-treatment (SVR12). Other virologic endpoints include virologic failure, SVR at Week 24 post-treatment (SVR24) and resistance.
Final results announced in February 2024 from the 60 patient lead-in cohort confirmed a 98% SVR4 rate across GT from 58 of 59 patients. These results include a patient with poor adherence who did not achieve SVR4 and exclude one patient who did not attend the Week 4 post-treatment follow-up. The SVR4 rate exceeded the protocol-defined efficacy criterion of ≥90% SVR4 for continuing the study.
As a result, patient enrollment was reinitiated for up to an additional 220 patients in the study, including patients with cirrhosis. Final SVR12 results from all patients enrolled in the Phase 2 study are anticipated in the second half of 2024.
In the lead-in cohort, very rapid viral kinetics were observed with viral load for each patient near or below the lower limit of quantification (LLOQ) at four weeks of treatment, which is supportive of an eight-week treatment regimen for the combination of bemnifosbuvir and ruzasvir. All 60 patients in the lead-in cohort achieved viral load below the LLOQ by the end of the eight-week treatment.
The combination of bemnifosbuvir and ruzasvir in the lead-in cohort was generally safe and well-tolerated and there were no drug related serious adverse events, no treatment discontinuations and adverse events were mostly mild.
In in vitro studies, bemnifosbuvir has been shown to be approximately 10-fold more active than sofosbuvir (SOF) against a panel of laboratory strains and clinical isolates of HCV GT 1–5. In vitro studies have also demonstrated bemnifosbuvir remained fully active against SOF resistance-associated strains (S282T), with up to 58-fold more potency than SOF. The pharmacokinetic (PK) profile of bemnifosbuvir supports once-daily dosing for the treatment of HCV. Bemnifosbuvir has been administered to over 2,100 healthy and HCV-infected subjects and has been well-tolerated at doses up to 550 mg for durations up 12 weeks.
Ruzasvir has demonstrated highly potent and pan-genotypic antiviral activity in preclinical (picomolar range) and clinical studies. Ruzasvir has been administered to over 1,200 HCV-infected patients at daily doses of up to 180 mg for 12 weeks and has demonstrated a favorable safety profile. Ruzasvir’s PK profile supports once-daily dosing.
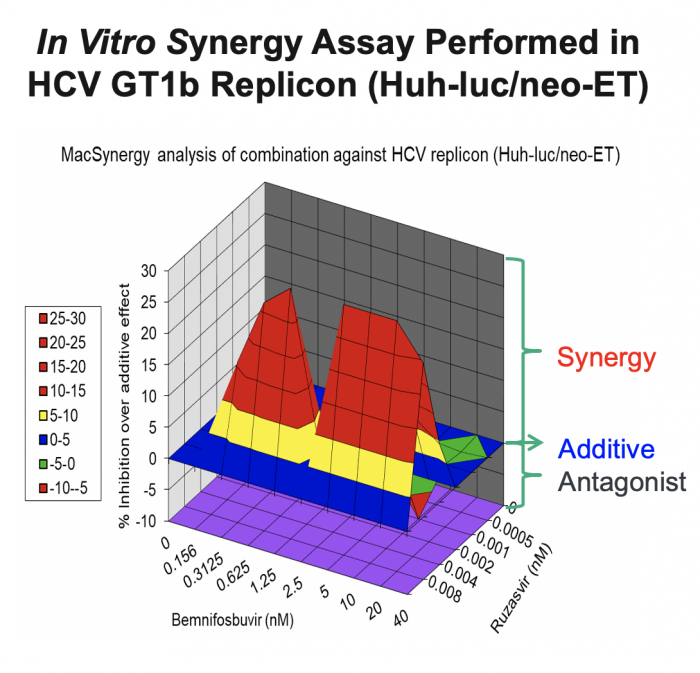
In support of the development of the combination of bemnifosbuvir and ruzasvir in patients, we conducted in vitro synergy experiments in HCV GT1b replicon assays (Huh-luc/neo-ET), where HCV replicon cells were treated with multiple concentrations of AT-511, the free base of bemnifosbuvir, and ruzasvir either alone or in combination. As shown in the figure below, these experiments demonstrated that the combination resulted in substantially greater inhibition of HCV replication than either agent alone, suggesting a synergistic antiviral effect between the two inhibitors.