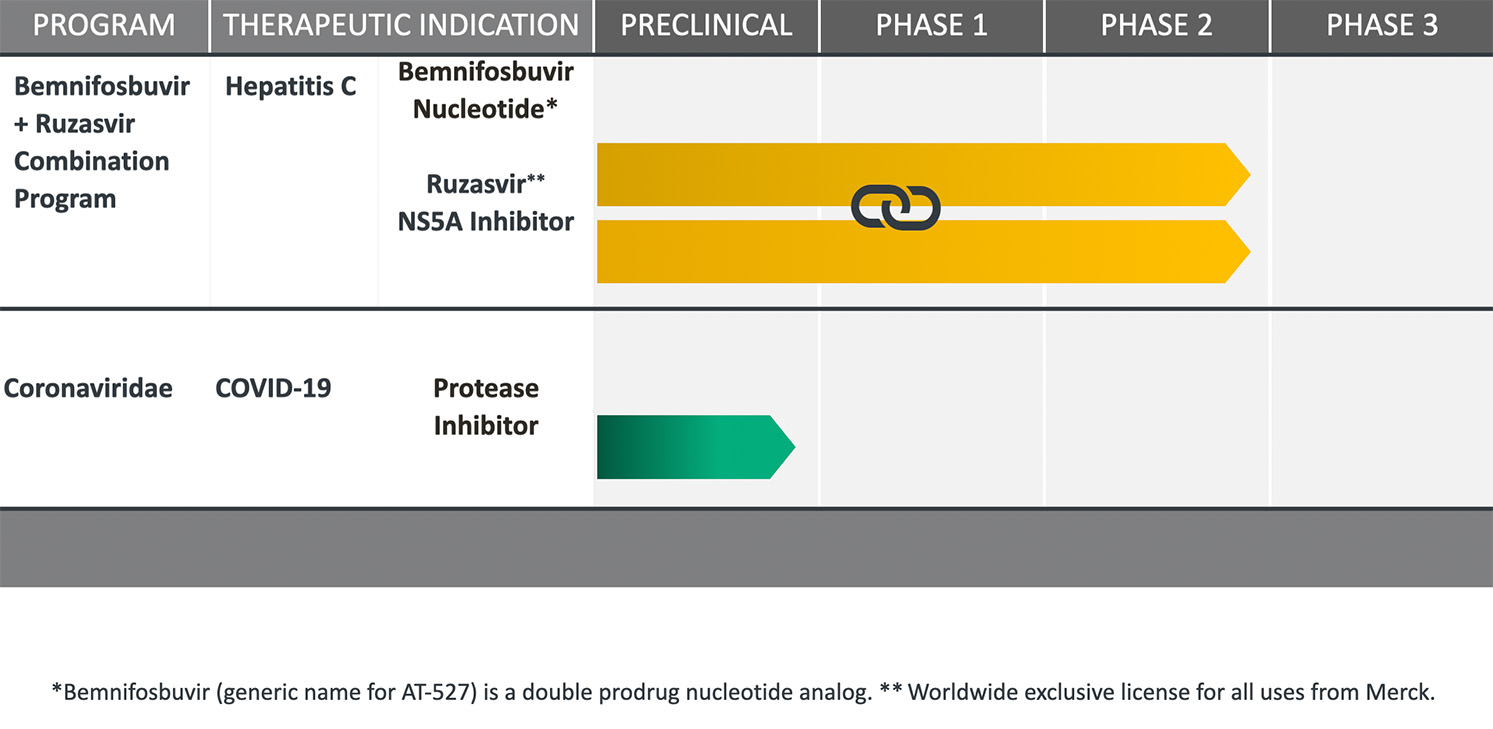
PIPELINE & PROGRAMS
Overcoming the limitations of existing therapies.
Relying on our extensive antiviral drug discovery and development capabilities, we are advancing proprietary oral small molecule therapeutics, comprised of highly selective inhibitors of viral replication.
Our product candidates, specifically targeting highly prevalent RNA viruses that cause serious human diseases, have been designed to overcome limitations of existing therapies.