COVID-19 AND BEMNIFOSBUVIR
Bemnifosbuvir has the potential to address key limitations of current therapies for COVID-19.
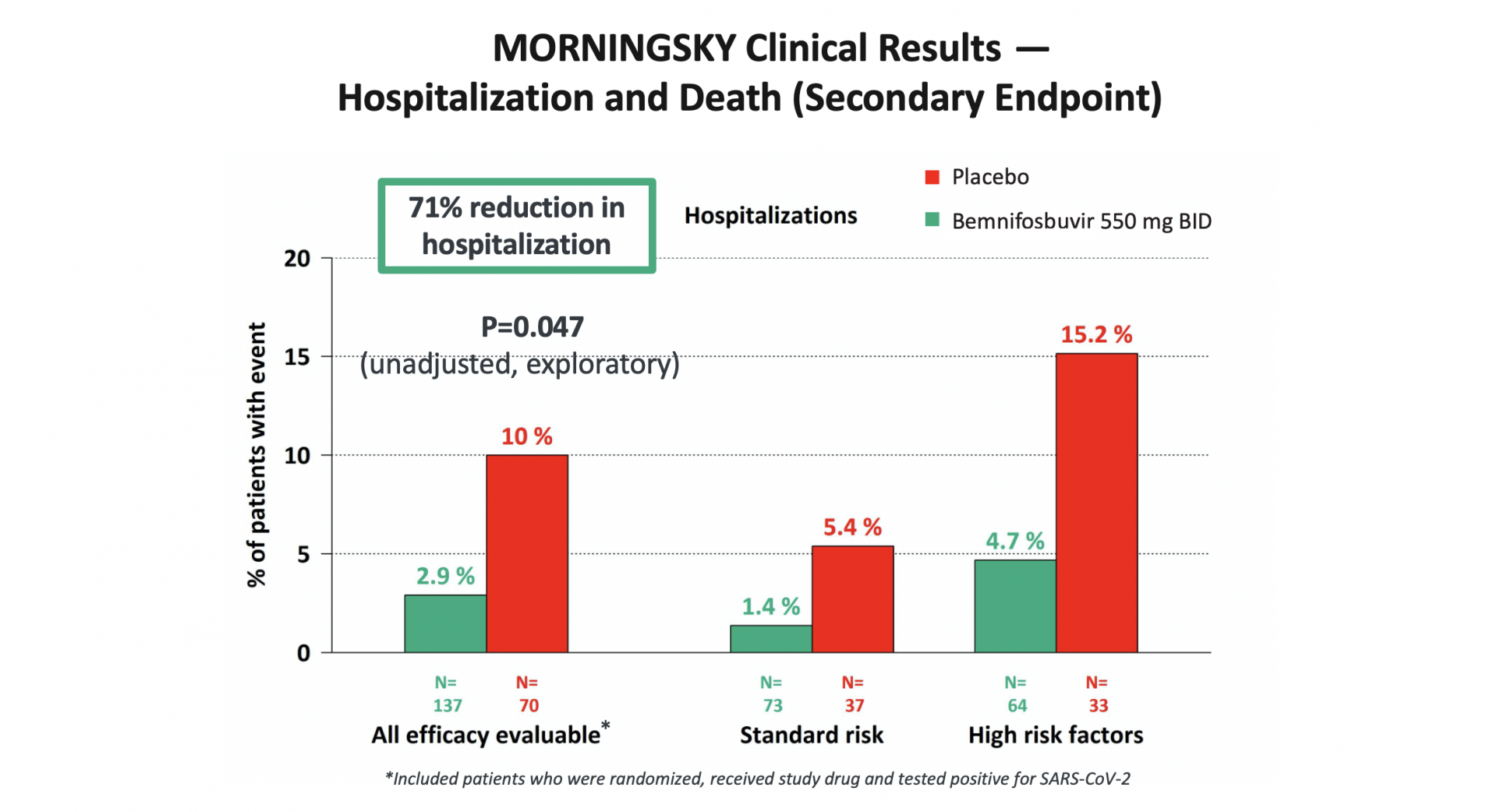
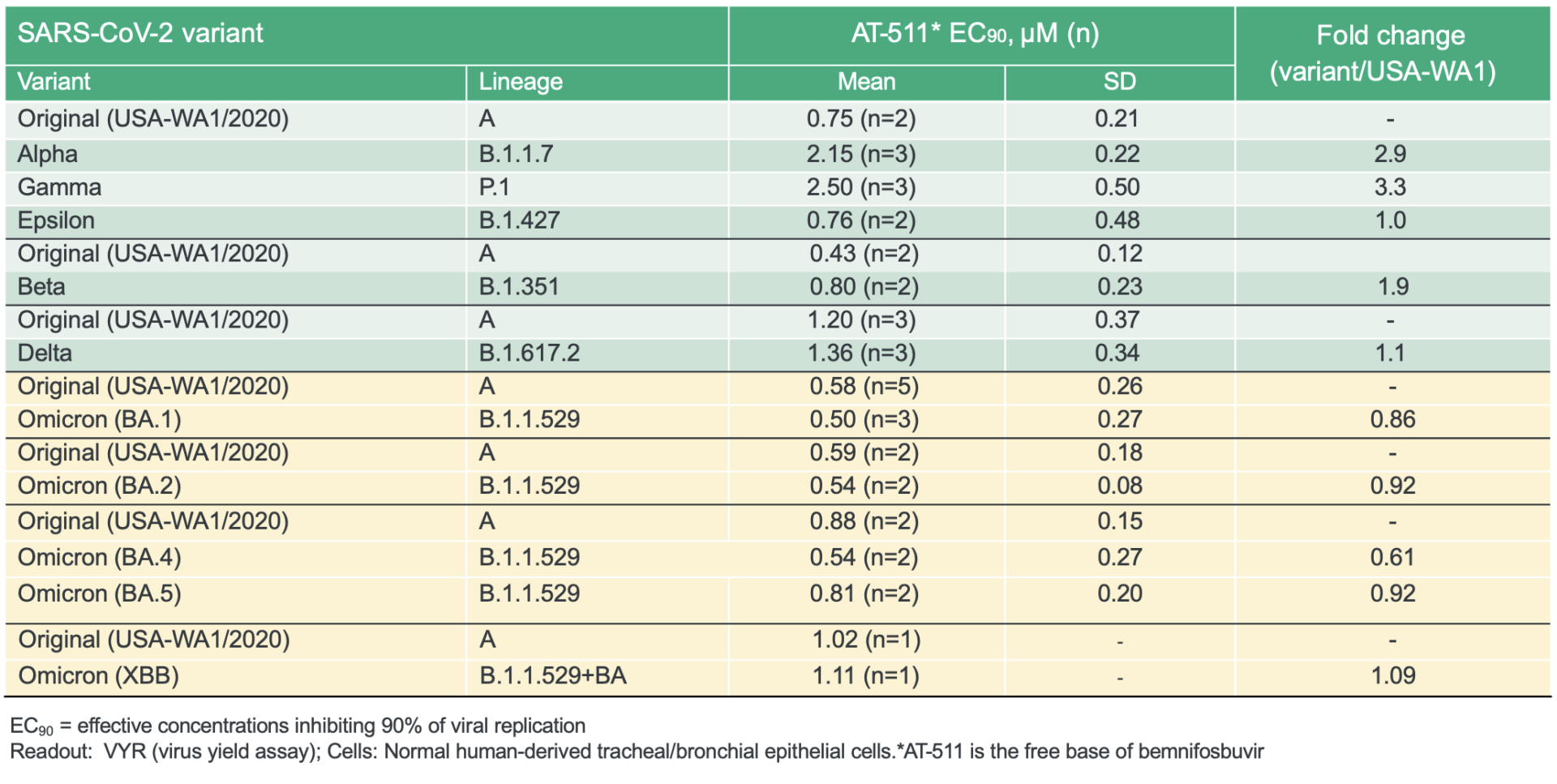
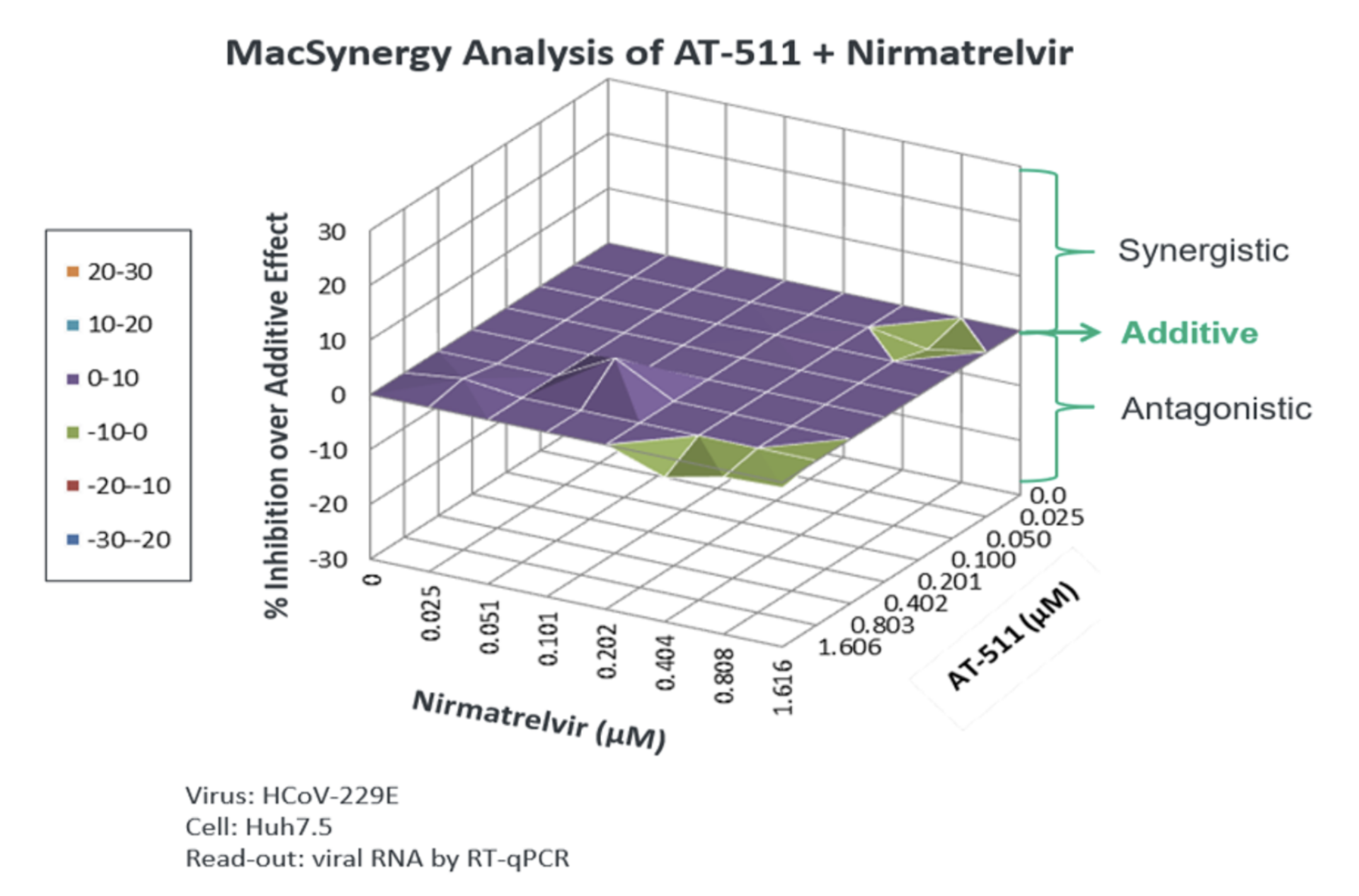
SARS-CoV-2 has proven to be endemic and will continue to be prevalent in society. Although vaccines play an important role in improving a patient’s chance of experiencing a milder form of COVID-19, the continuing ability of SARS-CoV-2 to evade vaccines and monoclonal antibodies combined with the limitations of current antivirals such as drug-drug interactions, highlight the urgent need for new, oral antiviral treatment options with improved profiles.
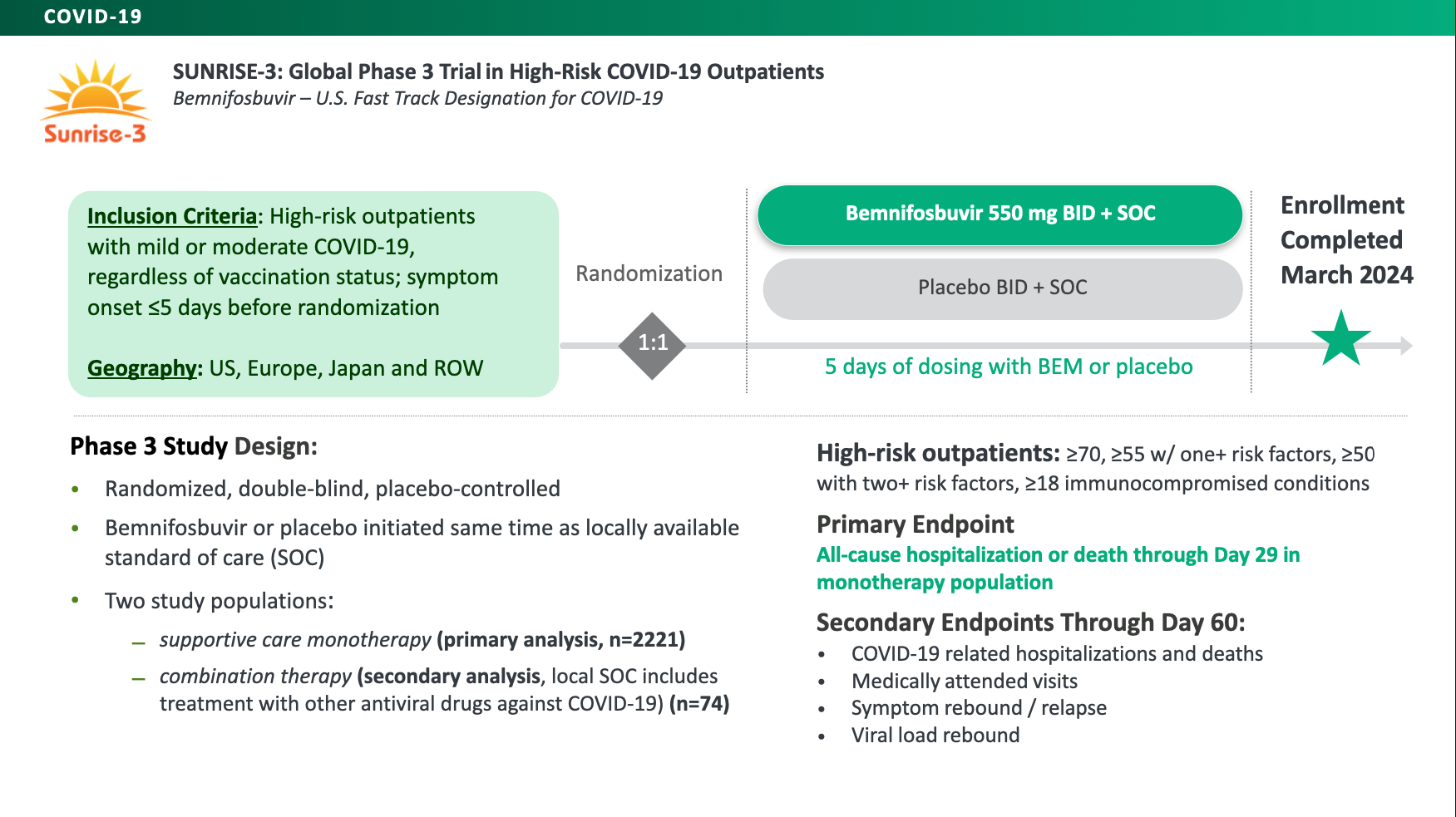
Bemnifosbuvir is an oral, direct-acting antiviral drug candidate that is being evaluated for COVID-19 in SUNRISE-3, a global Phase 3 registrational trial. In March 2024, Atea completed full enrollment of SUNRISE-3 with 2,221 high-risk patients in the bemnifosbuvir monotherapy cohort and 74 high-risk, patients in the combination therapy cohort.
Our most advanced product candidate, bemnifosbuvir, is an oral direct-acting antiviral, which is being developed as monotherapy and potential combination therapy for COVID-19. Our COVID-19 strategy for bemnifosbuvir focuses on the current unmet medical needs of high-risk patients. Our goal is to deliver a safe, effective and convenient treatment option for people that remain vulnerable to hospitalization or death.