HCV is a blood-borne, positive sense, ssRNA virus, primarily infecting cells of the liver. HCV is a leading cause of chronic liver disease and liver transplants and spreads via blood transfusion, hemodialysis, and needle sticks. In the US, injection drug use accounts for approximately 60% of all new cases of HCV. Diagnosis of HCV is made through blood tests, including molecular tests that allow for the detection, quantification and analysis of viral genomes and the classification of an infection into specific viral genotypes. Hepatitis C becomes chronic hepatitis C in 75% to 85% of acute cases, with an incubation period lasting from two to 26 weeks.
HCV is classified into seven genotypes and 67 subtypes, with genotype 1 being responsible for more than 70% of HCV cases in the US. Patients with HCV are also classified by liver function status: compensated cirrhosis (liver scarring) denotes those patients that do not yet have impaired liver function, while decompensated cirrhosis describes patients with moderate to severe liver function impairment.
According to the WHO, an estimated 58 million people globally have chronic HCV infection, with about 1.5 million new infections occurring per year. Based on the most recent HCV surveillance report (2020) published by the CDC in 2022, a total of 107,300 newly chronic hepatitis C cases were reported in 2020. Approximately 290,000 people die every year from HCV related liver diseases, with the majority of deaths related to cirrhosis and hepatocellular carcinoma.
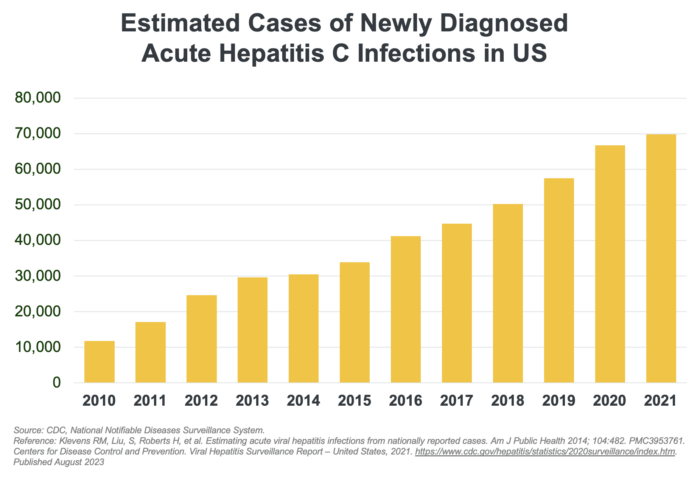
The incidence rate of acute HCV as reported through 2020 more than doubled since 2013 (124% increase). However, there is a wide gap between the number of reported cases versus estimated cases. Most individuals who become infected with HCV remain unaware that they are infected because HCV can go undetected until the condition progresses to symptomatic disease or until specific clinical tests are performed to confirm diagnosis. Consequently, cases are unreported, skewing actual disease prevalence rates. The burden of underreporting is realized when high medical expenditures (comorbid treatment costs, liver transplants) and mortality rates from advanced chronic liver disease do not proportionally align with reported prevalence rates.
Despite the availability of direct acting antivirals, there remains a large, underserved, HCV patient population which continues to grow in the US. A large portion of this increase in incidence is attributable to the opioid crisis, IV drug use and HCV reinfection.
The US HCV prevalence is expected to continue to remain steady over the coming years as rising HCV incidence offsets the number of new patients treated. It is estimated that a substantial global market for HCV therapeutics will exist to 2050 and beyond. In 2022, reported net revenue was approximately $3.5 billion in global sales, with approximately 50% attributable to the US.
A combination of bemnifosbuvir and ruzasvir has the potential to offer a differentiated short duration, pan-genotypic protease-sparing regimen for HCV-infected patients with or without cirrhosis.